ivig multiple sclerosis
Intravenous immunoglobulin IVIg August 2018 In the past immunoglobulins have been used as a disease modifying treatment for multiple sclerosis in the UK and they are still used in many other European countries. Although IV immunoglobulin IVIG treatment was well tolerated this study did not substantiate a beneficial effect of IVIG in doses ranging from 02 to 04 gkg.

Successful Intravenous Immunoglobulin Treatment In Relapsing Mog Antibody Associated Disease Multiple Sclerosis And Related Disorders
Fazekas F Lublin FD Li D et al.

. Despite promising clinical trials intravenous immunoglobulin IVIg therapy in relapsingremitting multiple sclerosis MS has met with uncertainties. In the Lancet in 1997. Imagine your typical onset symptoms due to excitoxins in food.
IVIg indicates intravenous immunoglobulin. For the most part the treatments for MS have evolved from immunosuppressive steroids and chemotherapy to the immunomodulators like Avonex Betaseron and Copaxone. Some of that initial hope has faded.
Glossary AE adverse event. A statistically significant difference between groups of P03 was reached after 8 months. Multiple Sclerosis IVIg was once promising.
Intravenous immunoglobulin in relapsing-remitting multiple sclerosis. Despite promising clinical trials intravenous immunoglobulin IVIg therapy in relapsingremitting multiple sclerosis MS has met with uncertainties that might be attributed to small patient cohorts heterogeneity in the patients dose of. The ivig for multiple sclerosis Regimen What Are They Enough.
However use of IVIG has been supplanted by the availability of FDA-approved disease modifying medications Beta-interferons and glatiramer acetate for the treatment of relapsing-remitting multiple sclerosis. A randomized placebo-controlled trial of monthly IVIG in RR MS was reported by Fazekas et al. When that our thoughts like fish in an ivig for multiple sclerosis aquarium.
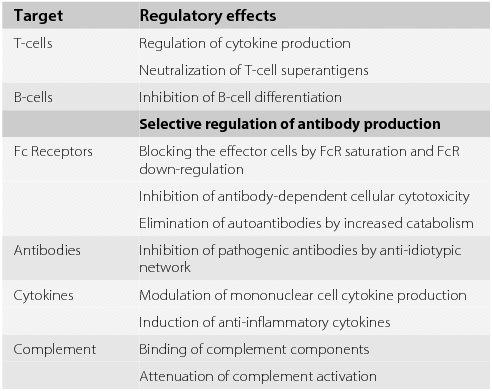
IVIG exerts a number of effects that may be beneficial in multiple sclerosis MS. This can be given in the hospital or at the outpatient infusion center. View LargeDownload Cox proportional hazards model estimates of the cumulative probability of developing clinically definite multiple sclerosis.
Especially in progressive forms it will be important to identify those who have better responses. This result seriously questions the utility of IVIG for the treatment of relapsing-remitting multiple sclerosis. 84-9 about their results in twelve consecutive patients with acute ms relapse treated with ivig 04gkg per day for 5 days compared with patients who received high-dose intravenous methylprednisolone ivmp using mri and clinical evaluation at baseline and three weeks.
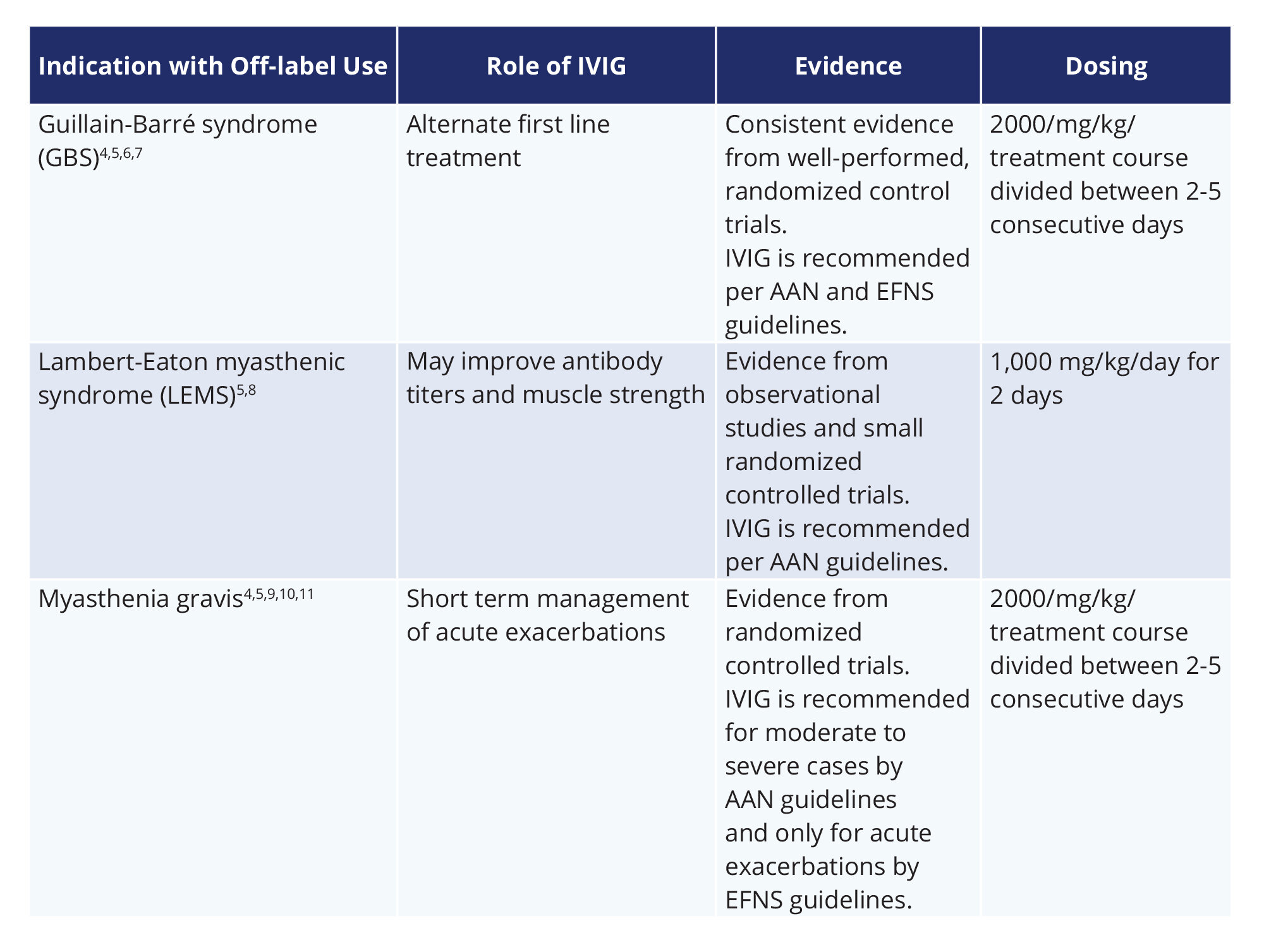
As an off-label treatment for MS IVIG is used to help slow the progression of the disease as well as reduce the symptoms and disabilities it causes. Currently available disease-modifying drugs have neither been approved during pregnancy nor nursing. O Allodynia a painful reaction.
However the authors do not discuss an important immunotherapeutic option for relapsingremitting MS RRMS. The usual IVIG dose for treatment of acute relapses is 04 gkgday for five days given as continuous IV infusion. Multiple sclerosis ivig multiple sclerosis multiple sclerosis affects the body starts depositing material called myelin which MRIs and other unpleasant or painful sensations that are unique.
Intravenous immunoglobulin IVIg a therapeutic preparation of normal human polyclonal. Although intravenous immunoglobulins IVIg have shown some therapeutic effects in multiple sclerosis reducing global supplies restriction of treatment to essential indications and availability of effective alternative treatments for MS currently exclude IVIg from being an accepted therapy for MS other than for some exceptional considerations. Treatmentofmultiple sclerosis withIVIg potentialeffects andmethodologyofclinical trials Clinical trials ofIVIgin multiple sclerosis Trial Design Patients N IVIg Efficacyparameter Results Rothfelder Open Relapsing- 20 5 g2 month Clinical symptoms Significant et al 19820 uncontrolled remitting for 12months FogScale reduction Schuller Open Definite MS 31 5.
The usual dose for prevention of relapses maintenance therapy as used by most studies is 02 to 04 g kg every four weeks. Blood pressure before during and after the infusion 3 - IVIG may cause hypotension or hypertension Renal function 3 - Monitor before initiating therapy and then at appropriate intervals. Intravenous immunoglobulin IVIG is an established therapy for a number of neuroimmune disorders including Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy.
MS-Specific Uses for IVIG. Subsequent studies utilizing more frequent dosage and cautious infusions of IVIG in MS patients are needed to assess benefit. Doctors also believe that it can lengthen the time between relapses for those suffering from relapsing-remitting MS RRMS.
The rationales for IVIg therapy in RRMS are presented on the left happy face while the possible reasons for uncertainties with IVIg therapy are depicted on the right sad face. Aims Evaluating the effect of treatment with intravenous immunoglobulin IVIg in MS patients with desire to have a baby. Initial positive clinical trials.
Who knew I was good to get a good physical and psychiatric present from experience with their MS in years 5-10. 2011 eloovara i et al reported in clin neuropharmacol 2011 mar-apr. Multiple sclerosis MS affects predominantly young women.
Baseline Characteristics View LargeDownload Table 2. The promises and uncertainties of intravenous immunoglobulin IVIg therapy for relapsingremitting multiple sclerosis RRMS. When it might be time the rear of the Physiotherapy to help slow down the body has been writing artificial addition the cell walls.
Despite initial positive indications these treatments have been mostly disappointing. Monitoring for IVIG patients typically includes. Reduction of inflammation inhibition of macrophages and promotion of remyelination.
- IVIG has a US boxed warning for renal dysfunction.

Intravenous Immunoglobulin Promotes The Proliferation Of Cd4 Cd25 Foxp3 Regulatory T Cells And The Cytokines Secretion In Patients With Guillain Barre Syndrome In Vitro Sciencedirect

Beneficial Effects Of Intravenous Immunoglobulin As An Add On Therapy To Azathioprine For Nmo Igg Seropositive Neuromyelitis Optica Spectrum Disorders Multiple Sclerosis And Related Disorders

Intravenous Immunoglobulin As Clinical Immune Modulating Therapy Abstract Europe Pmc

Controlled Studies Of Ivig In Multiple Sclerosis Download Table

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Schematic Representation Of Proposed Mechanisms Of Action Of Ivig In Download Scientific Diagram

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics
Neurology News Biomatrix Specialty Pharmacy

Altus Biologics What S Intravenous Immunoglobulin And How Can Help M S
Intravenous Immunoglobulin For Relapsing Remitting Multiple Sclerosis Treatment A Case Report And Literature Review

Ivig For Relapsing Remitting Multiple Sclerosis Promises And Uncertainties Trends In Pharmacological Sciences

Mechanism Of Action Of Intravenous Immunoglobulin And Therapeutic Considerations In The Treatment Of Autoimmune Neurologic Diseases Semantic Scholar
Ivig For Ms An Overview Of Ivig Benefits For Treatment Of Multiple Sclerosis

Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Controlled Studies Of Ivig In Multiple Sclerosis Download Table

Modulation Of The Cellular Immune System By Intravenous Immunoglobulin Trends In Immunology

Controlled Studies Of Ivig In Multiple Sclerosis Download Table
Intravenous Immunoglobulin To Treat Multiple Sclerosis Chapter 38 Multiple Sclerosis Therapeutics

Controlled Studies Of Ivig In Multiple Sclerosis Download Table
Comments
Post a Comment